Innovation
The Meiji Group promotes innovation through our synergy of food and pharmaceutical expertise. Our research into health and human longevity is groundbreaking and always follows the highest ethical standards.
Wellness Science Labs
Incorporating Technologies from Inside and Outside Meiji Group to Create a New Generation of Wellness.
Technology Development That Drives Innovation

Wellness Science Labs was originally established in 2019 as the Co-Creation Center, with the aim of creating synergy between the Meiji Group’s two core businesses of food and pharmaceuticals. In 2023, it then reaffirmed its mission to realize both the “new challenges in the health value domain” outlined in the Meiji Group 2026 Vision and the “contribution to addressing social issues” contained in the Meiji Group 2026 Sustainability Vision. That year, the center was re-conceived as Wellness Science Labs, as part of a commitment to actualize the strategies contained in the Visions through our technology-driven solutions. The reborn Wellness Science Labs now serves as the hub of the Meiji Group’s technology development—bridging and integrating the technologies of our two core business domains, food and pharmaceuticals.
Wellness Science Labs leads our efforts to build an innovation platform for the entire Meiji Group. Towards this goal, initiatives include enhancing the medium- to long-term technological development strategy for the next 10 years; exploring new business areas utilizing the diverse technologies and know-how cultivated by our group; and pioneering new business endeavors from technological seeds.
Another function of Wellness Science Labs is to further strengthen the Meiji Group’s business base by enhancing research and technology development in our areas of competence and expertise, such as maternal and child nutrition and the human microbiome.
Food and Pharmaceutical Businesses Combined with Open Innovation to Pioneer New Domains
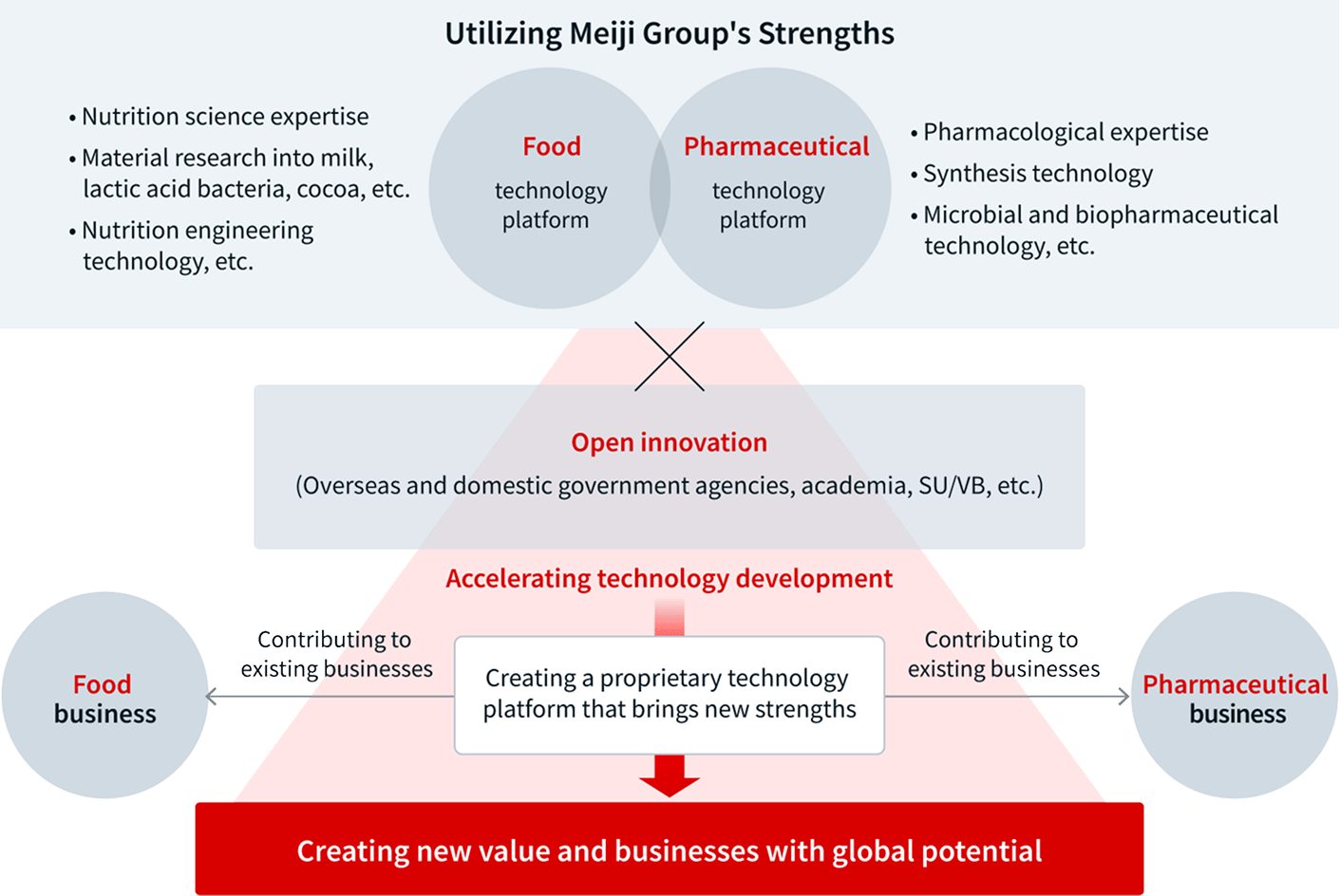
To pioneer new business domains, it is critical that we not only combine the strengths and technological assets of the Meiji Group’s food and pharmaceutical businesses, but also proactively introduce and incorporate ideas and technologies from beyond our organization. To accelerate such open innovation, we are partnering with external entities and linking those partnerships to the creation of innovative and competitive new businesses.
Strengths of Meiji Group
| Food Business Strengths | Nutrition science expertise; material research into milk, lactic acid bacteria, cocoa, etc.; Nutrition engineering technology, etc. |
|---|---|
| Pharmaceutical Business Strengths | Pharmacological expertise; synthesis technology; microbial and biopharmaceutical technology, etc. |
| Technology Assets | Microbial control; process technology; evaluation technology |
Over recent years, the majority of the world’s cutting-edge technologies have emerged from venture businesses and startup companies. Accordingly, partnerships with such companies have become increasingly important in accelerating technology development focused on societal application and the creation of new value.
In addition to partnering with domestic and overseas universities and public research institutes, Wellness Science Labs also proactively pursues investment and collaboration opportunities with startups and venture companies both in Japan and across the world. This approach increases the speed of technology development while contributing to the creation of a new and unique innovation platform within our group. Moving forward, we will leverage the Meiji Group’s existing expertise while tapping into open innovation, to create new strengths for the group as we challenge ourselves to create new businesses and generate new value with truly global potential.
Open Innovation
The Meiji Group creates innovative technologies, products, and business models by investing in venture companies and collaborating with various organizations such as companies, research institutes, universities, and hospitals.
Meiji Holdings Invests in New Protein Fund by Big Idea Ventures
Big Idea Ventures LLC was established in 2018 as a venture capital fund with offices in New York, Singapore, and Paris. The fund mainly invests in food ventures related to SDGs. In 2019, Big Idea Ventures formed the New Protein Fund to invest in startups developing technology in areas such as plant-based proteins and proteins derived from cultured cells. The fund will also function as an accelerator by providing expertise and manufacturing support.
Meiji Holdings Participates in Business Accelerator Program, TECH PLANTER
Organized by Japanese company Leave a Nest Co., Ltd., TECH PLANTER is an initiative targeted to individuals or teams before incorporation phase, to discover and nurture business seeds in the deep-tech field. TECH PLANTER supports business development and R&D by combining issues, technologies and assets brought by the entry team and by partner companies in a specific technology area. Meiji Holdings participates in three areas: food technology, bio-healthcare technology, and medical technology.
Joint Research Explains Universal Correlation between Protein Intake and Muscle Gain
Meiji Co., Ltd. has conducted joint research with Japan's National Institutes of Biomedical Innovation, Health and Nutrition. As a result of a meta-analysis* using a total of 5,402 study participants from 105 articles, it was clarified for the first time in the world that protein intake significantly increases muscle mass with or without resistance training. In particular, it is a remarkable finding that even a daily intake of 0.1 g of protein per kg of body weight leads to an increase in muscle mass.
Reference: Tagawa R, et al. Nutr Rev. 2021 Jan; 79 (1): 66-75.
Joint Research Establishes Link between Milk Protein and Muscle Mass in Older Adults
Meiji Co., Ltd. has conducted joint research with Obihiro University of Agriculture and Veterinary Medicine in Japan. In a clinical trial involving 122 healthy adults, it was confirmed that continuous intake of a beverage containing 10 g of milk protein per day for 6 months under low to medium intensity exercise training for about 10 minutes significantly increased the muscle mass of adult men and women aged 60 years or older. A small intake of 10 g per day is less burdensome, and the continuation rate for 6 months is 97.5%, indicating that it is a nutritional strategy that is easy to incorporate into daily life.
Reference: Nakayama K, et al. Eur J Nutr. 2021 Mar; 60 (2): 917-928.
Joint Research Confirms Positive Health Outcomes of Ingesting Yogurt Fermented with Lactic Acid Bacteria Strain
Meiji Co., Ltd. has conducted joint research with Institute of Community Life Sciences Co., Ltd. A clinical trial was held of 961 adult women working in medical institutions. In the experimental group that ingested yogurt fermented with our own lactic acid bacterium Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 strain once a day for 16 weeks, the quality of sleep and QOL (overall health and vitality was significantly improved, and constipation symptoms significantly suppressed compared to the control group (non-intake).
Reference: Kinoshita T, et al. BMC Gastroenterol. 2021 21 (237)
Joint Research Confirms Blood Sugar Decrease After Ingesting Yogurt Containing Lactic Acid Bacteria Strain
Meiji Co., Ltd. has conducted joint research with the University of Tokyo. A clinical trial was held of 130 pre-diabetic adults. In the experimental group that ingested yogurt containing Lactobacillus plantarum OLL2712 strain, which is our own lactic acid bacterium, once a day for 12 weeks, HbA1c was significantly decreased as compared with the control group (intake of yogurt not containing OLL2712 strain). In addition, a significant increase in insulin resistance was observed in the control group, but it was maintained normal in the experimental group.
Reference: Toshimitsu T, et al. Nutrients. 2020 Jan 31; 12 (2): 374
Joint Research Establishes Energy and Task Performance Benefits of Ingesting Jelly Drinks
Meiji Co., Ltd. has conducted joint research with Aoyama Gakuin University in Japan. A clinical trial took place of male students aged 21 to 26 years. By ingesting jelly drinks containing amino acids, etc., compared to when not ingesting, (1) significant suppression of performance deterioration for tasks that require concentration, (2) promotion of active efforts to tackle tasks, and (3) significant improvement of psychological state (comfort, arousal, vitality, and refreshment) was observed. This result was presented at the 26th International Symposium on Artificial Life and Robotics (AROB) 2021.
Joint Research Conducted Using Newly Developed 4D Swallowing Simulator
Meiji Co., Ltd. has conducted joint research with Musashino Red Cross Hospital at the request of the Consumer Affairs Agency of Japan. The goal was to prevent choking and aspiration of children by utilizing Swallow Vision®, the world's first 4D swallowing simulation system jointly developed by Meiji and Musashino Red Cross Hospital. The effects of the shape from the oral cavity to the respiratory tract, the shape of food, and the posture and movement during swallowing on choking and aspiration were evaluated. A warning video created based on this research has been released on the Consumer Affairs Agency website, contributing to public awareness.
Launch of Europe's First Cube-type Infant Formula, through a Business Alliance with Danone
A cube-type version of Danone's main brand of infant formula Aptamil was released in the United Kingdom through a business alliance between Meiji Co., Ltd. and Danone S.A. Cube-type infant formula is a unique technology developed by Meiji and is highly expected as a product that can support child-rearing even in the United Kingdom. Danone also plans to roll out cube-type infant formula in other European countries after 2022. Meiji will further expand its business in Europe and aims to achieve overseas sales of 10% as set forth in its 2026 vision.
Drug Discovery for a Breakthrough COVID-19 Therapy Based on Ivermectin Derivatives
In May 2021, Meiji Seika Pharma commenced joint research and development with Kitasato Research Institute, with the aim of discovering therapeutic drugs based on next-generation ivermectin derivatives and establishing a foundation for antiviral drugs. Ivermectin derivatives have anti-inflammatory and immunomodulatory effects in addition to antiviral effects, and have the potential to not only treat COVID-19 but also prevent post COVID-19 syndrome.
Development of Inactivated Vaccine (COVID-19)
Meiji Group company KM Biologics is developing an inactivated vaccine against COVID-19 in collaboration with the Institute of Medical Science at the University of Tokyo, the National Institute for Health Risk Management, the National Institute of Infectious Diseases, and the National Institutes of Biomedical Innovation, Health and Nutrition, among others. By combining expertise from several organizations renowned for advanced research in the fields of infectious diseases, virology, and immunology, the joint project is working on various initiatives to prepare for future pandemics, including COVID-19.
Joint Research in the Field of Advanced Medical Care
The Meiji Group is conducting joint research with the Foundation for Biomedical Research and Innovation at Kobe with the aim of developing therapeutic agents for autoimmune diseases such as rheumatoid arthritis. We have already succeeded in creating candidate antibodies for the treatment of autoimmune diseases and applying a patent. We are now in process oftesting their safety and efficacy before conducting clinical trials.

Creative Lab for Innovation in Kobe of Institute of Biomedical Research and Innovation
©Kobe Urban Promotion Service Co.,Ltd.
Harnessing Digital Transformation (DX) for Prevention of Depression
Meiji Seika Pharma has signed a joint research agreement with Hiroshima University to research, develop and socially implement digital platforms for preventing depression. This DX initiative applies Hiroshima University's proprietary research findings on the disease.
Ethical Considerations in R&D
As a leader in food and health, the Meiji Group conducts research activities in pursuit of new health value for consumers and patients. Meiji Group research and development is conducted in compliance with relevant laws, policies of the relevant ministries and agencies, and internal rules for product quality, efficacy and safety.
Ethical Considerations in Research Using Human Biological Materials
The Meiji Group conducts objective and thorough investigations of scientific and ethical issues prior to engaging in research using biological materials from humans, such as tissues, cells, blood, or genes, as well as information from human subjects. Recently, basic research and regenerative medical research utilizing ES cells, iPS cells, and other biological materials from human subjects has been expanding at a rapid pace. All of our research complies with national guidelines and guidance* in the use of such human samples and information for research purposes.
Ethical Considerations in Animal Testing
The Meiji Group places great importance on animal protection and welfare. Experiments are based on the 3Rs principle of reduction (using fewer animals), replacement (seeking experiments that do not use animals) and refinement (mitigating animal suffering). We only conduct animal testing after receiving approval from a laboratory animal ethics committee, and Meiji Group animal experimentation undergoes evaluations and certifications from external organizations.
Our Food segment will not fund, conduct, or commission any tests on animals for health claims that are not required by law.

Handling of Biohazards and Living Modified Organisms
The Meiji Group obeys strict rules and procedures for safe handling of biohazard materials including pathogenic microorganisms, based on the WHO Laboratory Biosafety Manual. In particular, the proper handling of pathogens and other materials regulated under the Infectious Disease Act1, the Act on Domestic Animal Infectious Diseases Control, and other laws is overseen by an internal expert committee to ensure that any such materials are handled in accordance with relevant laws, which includes completing the relevant approvals and filings.
We have also established internal rules and an internal committee to oversee handling of living modified organisms and gene recombination in conformance with the Cartagena Act2. The internal committee carries out the proper checks to ensure experiments using living modified organisms are conducted in line with the standards provided in the Cartagena Act.
1.Infectious Disease Act: Act on the Prevention of Infectious Diseases and Medical Care for Patients with Infectious Diseases
2.The Cartagena Act: Act on the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms
Ethical Considerations Related to Medical Research Involving Human Subjects
The Meiji Group is compelled to confirm the safety and effectiveness of our products through medical research that involves human subjects (clinical trials and clinical research). In conducting clinical trials and clinical research, we exercise respect for the sanctity of life and respect for human rights in conformity with the Declaration of Helsinki1, complying with related laws and regulations2 of each country and region and conducting ethical medical research.
When conducting medical research involving human subjects, the Meiji Group gives the utmost consideration to protecting human rights and ensuring the safety of the participants. At the same time, we strive for transparency in our research and to ensure scientific propriety, independence, and reliability. These ethical and safety issues are continually examined by our research ethics committee and institutional review board.
1.The Declaration of Helsinki: Standards for ethical medical research involving human subjects
2.Japanese guidelines, including the Ethical Guidelines for Medical and Health Research Involving Human Subjects
Clinical Trial Information Disclosure
Clinical trials are disclosed in Clinical Trial Registries accessible to the public free of charge in line with applicable regulations.
Links to Clinical Trial Registries Portal
