Compliance
Basic Views
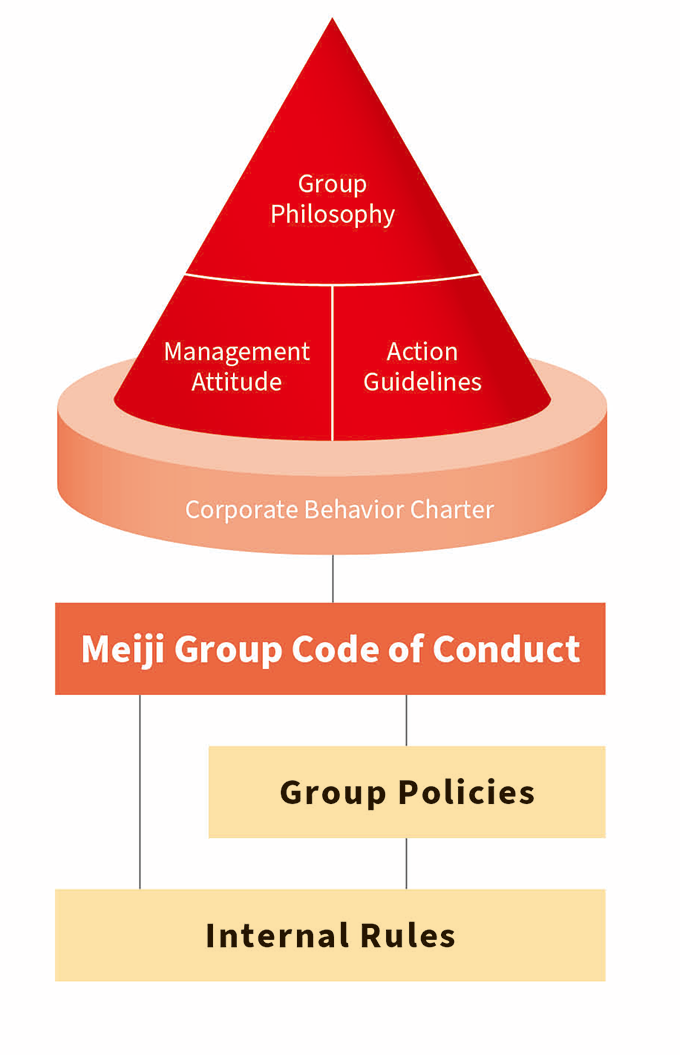
The Meiji Group complies with the laws, regulations and social rules of each country in order to ensure fair and free competition as well as proper and transparent transactions. To increase awareness and strengthen compliance further, we established internal regulations based on our Corporate Behavior Charter and the Meiji Group Code of Conduct and work to improve internal training. We conduct business holding ourselves to high ethical standards and shall continue to develop to be a company group trusted by society.
Management System
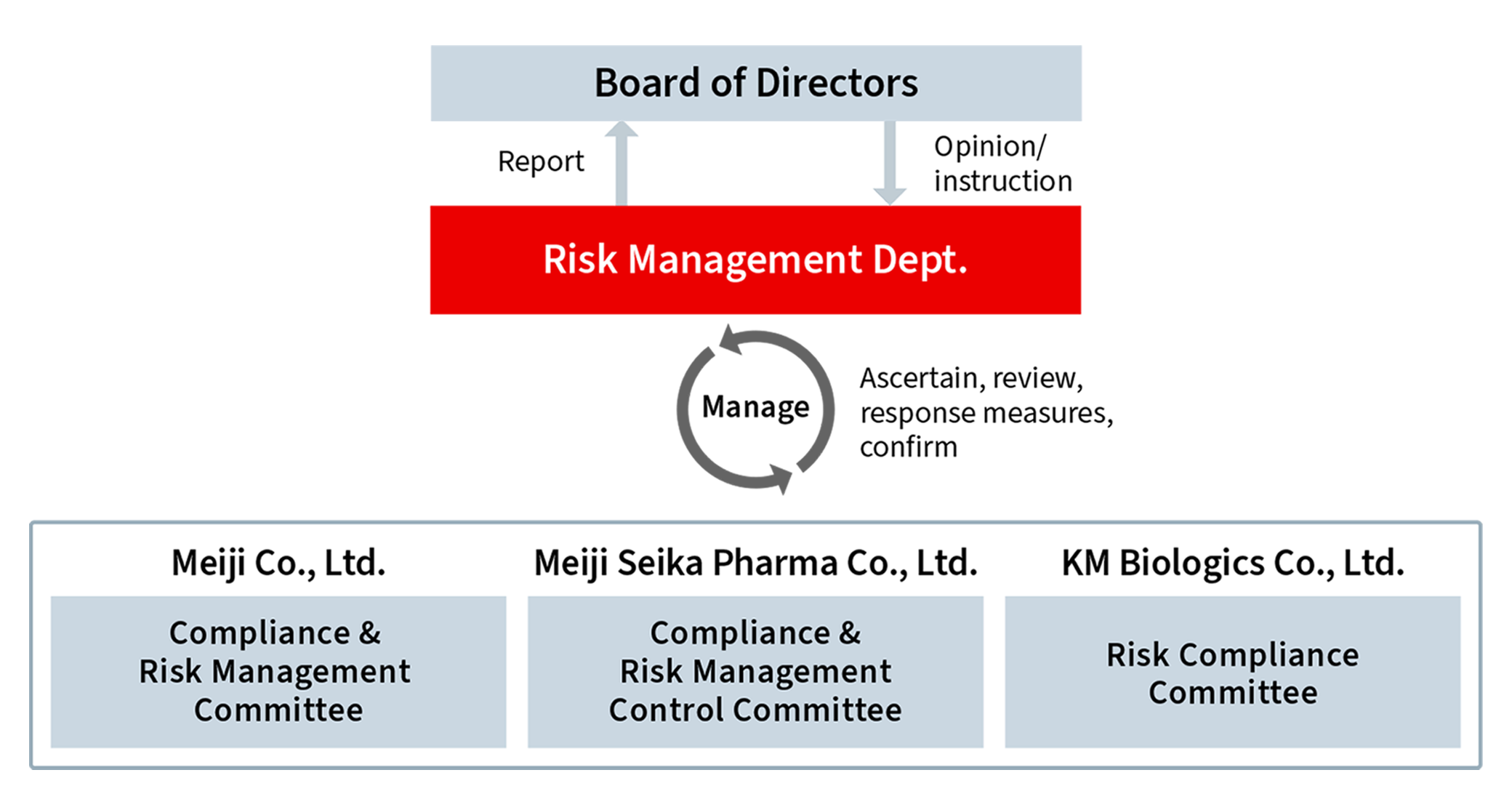
The Meiji Group has established an effective compliance management system by developing related regulations, such as the Compliance Regulations and setting up relevant committees within each Group company.
Compliance awareness
The Meiji Group has established the Meiji Group Code of Conduct which sets forth concrete values, concepts, and standards for implementing the Corporate Behavior Charter and clarifies the actions required of each and every executive and employee of the Meiji Group. The Meiji Group Code of Conduct has been translated into English, Chinese, Spanish, Thai, and Indonesian, and is being disseminated to employees not only in Japan but also overseas. To deepen understanding of the Meiji Group Code of Conduct and raise compliance awareness among all executives and employees, we conduct various compliance education and training programs, publish and disseminate in-house educational materials, and conduct compliance surveys. In FY2023, we conducted employee training aimed at raising awareness and implementing the Meiji Group Code of Conduct for all Group employees in Japan and overseas Group company secondees.
Anti-Corruption
The Meiji Group formulated Meiji Group Anti-Corruption Policy in order to remain a transparent and sound corporate group that is trusted by society. In May 2019, we became a signatory to the UN Global Compact and we comply with Principle 10: Business should work against corruption in all its forms, including extortion and bribery. We translated this policy into English, Chinese, Spanish, Thai, Indonesian and Hindi to raise employee awareness, not only in Japan but overseas as well. We are continuing our efforts to deepen employees' understanding of the Meiji Group Code of Conduct and Anti-Corruption Policy through in-house training, e-learning, and awareness-raising activities on our internal portal site. In FY2024, we conducted an e-learning program for approximately 13,000 employees, including all Group employees in Japan and those seconded to overseas Group companies. This program aimed to raise awareness of our Code of Conduct and Anti-Corruption Policy. It comprehensively covered the prohibition of corrupt practices such as bribery, abuse of authority, money laundering, and insider trading, emphasizing the importance of integrity through specific case studies. The participation rate of this e-learning program was 93.5%.
Meiji Group Anti-Corruption Policy
The Meiji Group regularly conducts operations audits of domestic and overseas Group companies. Operations audits include audits of compliance-related matters, such as fraud and corruption prevention as stipulated in the Meiji Group Anti-Corruption Policy or the Meiji Group Code of Conduct. In the last three years from FYE March 2024, we have completed operations audits for 65% of our Group companies. For overseas Group companies, in addition to operations audits, we are conducting audits specifically designed to reduce fraud, corruption and other management risks (referred to as "governance audits"). In the last three years from FYE March 2024, we have completed governance audits for 10 companies. Governance audits are used to confirm frameworks and management related to fraud prevention, including promotion of Meiji Group Policy awareness, anti-corruption, separation of duties, whistleblowing systems, and risk management systems. The use of external experts ensures audit efficiency and efficacy. Audit results are reported to audited companies and also shared with the Group CEO and other relevant officers, corporate auditors, and each operating company. Through these initiatives, we are working to strengthen our internal systems, and fraud checks and prevention.
Compliance Breaches
Once a breach of compliance becomes evident, we will promptly take corrective action and take appropriate action (such as disciplinary action in accordance with the Rules of Employment, including pay reduction, demotion, etc.) against those involved in such breaches.
The number of disciplinary actions for compliance breaches in FYE March 2025 was as follows.
Number of disciplinary actions taken for compliance breaches (FYE March 2025)
| Breakdown | incidents |
|---|---|
| Corruption or Bribery | 0 |
| Harassment | 0 |
| Labor | 1 |
| Quality | 0 |
| Information Management | 0 |
| Conflicts of Interest | 0 |
| Money Laundering or Insider trading | 0 |
| Accounting Fraud | 4 |
| Others | 9 |
Compliance Counseling Desk
The Meiji Group has established measures to promote the prevention and early discovery of any violations of legal code, the Meiji Group Code of Conduct, and the Meiji Corporate Behavior Charter. We maintain an internal whistleblowing system for employees that includes an internal hotline as well as a consultation desk staffed by an external attorney or other qualified professional to ensure independence from Company management.
For overseas Group companies, we have established local reporting channels that allow directors and employees to report in the language they use. We also have a reporting channel in Japan to receive such reports.
The Meiji Group explicitly prohibits the unjust treatment of whistleblowers in Company rules and regulations concerning internal reporting. We also protect whistleblowers by enabling anonymous reporting. Reported information is handled strictly as confidential information and is shared with relevant committees at each company. These committees then examine reported information and respond accordingly.
The Board of Directors of Meiji Holdings regularly receives reports on the operating status of the compliance systems and activities of each operating company and the operation of the internal reporting system, and supervise the operation of such systems.
The Meiji Group provides employees with cards containing whistleblowing hotline information. This information is also posted to our intra site to promote awareness of the whistleblowing system among employees.
In FYE March 2025, there were 234 reports and consultations throughout the Group.
An Ethical and Transparent Pharmaceuticals Company
Responsible promotion of ethical pharmaceuticals
The Meiji Group Pharmaceutical business embraces the mission of contributing to improving the health and welfare people around the world by developing innovative, highly effective, and safer pharmaceuticals. Critical to achieving this mission is maintaining strong ethical practices and transparency throughout our business activities. Furthermore, we must ensure appropriate interactions with researchers, medical professionals, and patient organizations, and engage in responsible promotional activities.
To ensure appropriate promotional activities, we maintain compliance with the Pharmaceutical and Medical Device Act and other relevant Japanese laws, and follow the laws, regulations, and guidelines of each country in which we engage in promotional activities for ethical pharmaceuticals.
In Japan, we used the Japan Pharmaceutical Manufacturers Association (JPMA) Code of Practice as the framework for creating our own Code of Practice. To supplement this Code, we also have outlined a Promotion Code, which reflects the principles of the Fair Trade Competition Code created by the Fair Trade Council of the Ethical Pharmaceutical Drugs Marketing Industry. We also established our Ethical Pharmaceutical Marketing Activity Regulations, which we based on the Ethical Pharmaceutical Marketing Activity Guidelines drafted by the Ministry of Health, Labour, and Welfare.
In our Promotion Code, we explicitly outline management duties and matters of compliance concerning promotional activities. Our Marketing Code for Ethical Pharmaceuticals outlines the organizational structure and role of the Marketing Supervisory Division, an entity that is independent of the Sales Division. This Code also outlines promotional procedures. When evaluating promotional materials, the Marketing Supervisory Division invites independent external experts from outside the Group to participate in their evaluation process.
Overseas, we conduct sales activities in compliance with promotion codes outlined by the respective industrial organizations of Indonesia, Thailand, and Spain where we have sales offices.
Employee education and internal audit concerning the promotion of ethical pharmaceuticals
To ensure the Group promotes are conducted in accordance with the Promotion Code and the Marketing Code for Ethical Pharmaceuticals, we regularly conduct training for all pharmaceutical sales division employees to ensure awareness.
To confirm compliance with the Promotion Code and the Marketing Code for Ethical Pharmaceuticals, an internal audit division conducts regular audits to confirm the adequacy of our compliance structure and activity processes, including promotional activities.
The Market Supervisory Division confirms the appropriateness of marketing representative (MR) activities by monitoring their activities, including accompanying MRs on sales visits to evaluate MR activities. The Market Supervisory Division also implements responses to MR activities as necessary.
Participation in industry initiative related to safe and responsible pharmaceuticals
To ensure the Group maintains membership in the following industry organizations as part of our efforts to ensure the safety of our pharmaceuticals. We partner with other companies towards establishing and promoting industry rules on matters such as the appropriate use of pharmaceuticals.
- The Federation of Pharmaceutical Manufacturers' Associations of Japan
- Japan Pharmaceutical Manufacturers Association
- Pharmaceutical Manufacturers' Association of Tokyo
- The Fair Trade Council of the Ethical Pharmaceutical Drugs Marketing Industry
- Council for the Proper Use of Medicine
- Japan Association of Vaccine Industries
- Japan Society of Quality Assurance
- Japan Blood Products Association
Transparency in corporate activities and relationships with medical institutions and patient organizations
Regarding interactions with researchers, medical professionals, patient groups, etc., each company has established its own Code of Practice for all directors and employees to ensure a high level of morality. The Pharmaceutical segment has also established Guidelines on Transparency of Relationships between Corporate Activities and Medical Institutions. Based on these guidelines, we disclose details of the academic research grants it provides.disclose details of the academic research grants it provides. These disclosures help illustrate the ways in which we contribute to progress in the life sciences, while adhering to the highest ethical standards.