Health and Nutrition
Contribute to Healthy Diets
Meiji's Nutritional Commitments
Since its founding more than 100 years ago, the Meiji Group has sought to contribute to society through nutrition, working to widen the world of “Tastiness and Enjoyment,” and to meet all expectations regarding “Health and Reassurance.” For the Meiji Group, “nutrition” means contributing to the resolution of health issues faced by all, and recognizing the importance of the happiness and the sense of fulfillment that eating tasty food can bring.
What does nutrition mean at Meiji? This important concept has now been enshrined in the Meiji Nutrition Statement, so that we can go on contributing to society for another 100 years. More specifically, we have made the following three commitments:
・Using milk, cocoa, and other natural ingredients, we provide nutrition that supports everyday good health.
・By adding value, we provide nutrition that satisfies deeper needs.
・Through nutrition, we will help to enrich the lives of consumers worldwide.
Initiatives to Combat Micronutrient Deficiencies and Undernutrition
Today, societies around the world are facing two seemingly contradictory problems. On the one hand, there is a rise in non-communicable diseases1 associated with overweight and obesity, which stem from overeating and unbalanced diets. On the other hand, there are increasing concerns about stunted growth, malnourishment, and frailty2, which stem from a lack of protein, dietary fiber, vitamins, and other nutrients.
If we wish to resolve such issues, it is vital that we make it easier for people to maintain nutritionally balanced diets. For this reason, we at the Meiji Group are developing products that contribute to the resolution of health and nutritional issues. At the same time, we are promoting initiatives aimed at using the Meiji Nutritional Profiling System to improve our products and provide information to our customers.
Formulating the Meiji Nutritional Profiling System (Meiji NPS)
In June 2023, the Meiji Group formulated the Meiji Nutritional Profiling System (Meiji NPS) after referring to international Nutritional Profiling System (NPS)*.
We aim to improve the nutritional value of our products by using Meiji NPS. We also aim to provide customers with clear information about these nutritional values to help them maintain even healthier diets.
Creation of Health-oriented Foods1 and Value-added Nutritional Foods2
We are helping resolve health and nutritional issues caused by micronutrient deficiencies and the double burden of malnutrition by leveraging the strengths of our Food and Pharmaceuticals businesses and our unique nutrition engineering technology to provide products that enable customers to consume the nutrients they need in a well-balanced manner.
 |
 |
 |
 |
 |
| Meiji Bulgaria Yogurt | Meiji Probio Yogurt R-1 | Chocolate Kouka Cocoa 72% | SAVAS Muscle Elite | Meiji MeiBalance |
Products That Support Portion Size Control
The Meiji Group offers a variety of products in different portion sizes (the same content in multiple amounts) to make it easier for customers to adjust the amount of food they eat at one time to suit their own needs.
By expanding the range of product choices with the addition of the small-volume product lineup, we will contribute to the realization of a healthy lifestyle for our customers by curbing excessive intake.
Creation of Foods with Our Environmentally and Socially Conscious Procurement Activities for a Sustainable Society
We are developing food products that contribute to the sustainability of raw material production bases, such as cocoa, raw milk, and lactobacillus, and support business continuity.
 |
 |
 |
||
| Meiji Oishii Gyunyu | Meiji Milk Chocolate | Meiji Hokkaido Tokachi series (yogurt and cheese) |
Promote Healthy Diets and a Healthy Food Culture
As a company that supports customers' healthy diets, the Meiji Group strives to popularize and raise awareness by providing products and disseminating information about healthy diets and dietary cultures.
Expanding Nutrition and Healthy Diet Education Activities
| FYE 3/2021 | FYE 3/2022 | FYE 3/2023 | FYE 3/2024 | FYE 3/2025 | |
| Japan | 9.7 | 18.8 | 25.5 | 28.3 | 29.3 |
Nutrition Education Initiatives in Japan
The Meiji Group began offering nutrition education in 2006, one year after Japan enacted a law concerning food and nutrition education. By explaining the value of nutrients, how food is produced, and the challenges faced by producers of milk, chocolate, and other products, the programs aim to encourage people to develop healthy eating habits and foster an appreciation and understanding of food.
In recent years, the Group has been targeting a broad range of age groups through its numerous education programs held at events it sponsors and at high schools, universities, workplaces, and public facilities for senior citizens. The programs focusing on health management, which has been attracting attention recently, have been especially popular among participants.
From FY2020, food and nutrition seminars were held online and attracted large numbers of participants. The online format also enabled greater coverage across Japan, including its island communities. Having received very positive feedback from participants of all age groups, the Group plans to offer even more seminars about food and nutrition as way to promote good health and well-being.
Overview of Activities
- Head office and eight workplaces around the country (in the cities of Sapporo, Sendai, Gunma, Tokyo, Nagoya, Osaka, Hiroshima, and Fukuoka)
- Nationwide organization with a staff of about 60 in charge of its nutrition education activities
- Participating students: 167,274 from 1,318 elementary and junior high schools (FY2024)
- Participating students: 144,587 from 1,420 elementary and junior high schools (FY2023)
- Participating students: 144,197 from 1,394 elementary and junior high schools (FY2022)
- Participating students: 122,917 from 1,253 elementary and junior high schools (FY2021)
- By the end of FY2020, lessons were held at over 10,000 elementary and junior high schools with more than one million students participating.
Supervisors for Our Programs
- For dairy nutrition: Tadao Saito, Professor Emeritus, Graduate School of Agricultural Science, Tohoku University
- For physiology: Hiroshi Nose, Professor, Department of e-Health Science, Shinshu University Graduate School of Medicine

Nutrition Education Initiatives in Overseas
In China, Meiji Dairies (Suzhou) implements an nutrition education for children focusing on yogurt. Through fun hands-on activities, the program teaches kids about dairy cows, milk, yogurt, and nutrition.
Research Focused on the Chewing—Swallowing Process
The Meiji Group conducts research focused on the chewing-swallowing process to support healthy and comfortable eating habits. At the core of this research is ORAL-MAPS®, a chewing process simulator, and Swallow Vision®, the world's only computer simulator of swallowing. We use these technologies to scientifically clarify the process by which food reaches a state where it can be swallowed by chewing and the mechanism of swallowing in order to support the joy of eating with the mouth.
We are also working on the development of a new simulation device. This device focuses on the action of sending food (bolus) from the mouth to the throat and esophagus just before swallowing. It helps to analyze the risk of the bolus blocking the airway. Furthermore, we will improve the accuracy of our research by establishing experimental methods for eating with the mouth, and we will also broaden the scope of future product development.
Nutrition Policies and Related Measures
We at the Meiji Group are developing measures based on various policies including the Meiji Group Policy for the Marketing of Breast-milk Substitutes, the Food Nutrition Labeling Policy and the Marketing Communication to Children Policy which were established in accordance with international guidelines.
- Meiji Group Policy for the Marketing of Breast-milk Substitutes
- Meiji Group Food Nutrition Labeling Policy
- Meiji Group Marketing Communication to Children Policy
Responsible Marketing of Breast-milk Substitutes (BMS)
Based on our recognition of breast-milk as the best nutrition for babies, we adopt the basic stance of recommending breastfeeding. Furthermore, we recognize the importance of the World Health Organization (WHO) ‘s International Code of the Marketing of Breast-milk Substitutes (WHO Code) and subsequent related resolutions by the World Health Assembly (WHA). The WHO recognizes the role of breast-milk substitutes (BMS) as the only safe and high-nutrient alternative to breast-milk when used appropriately. As a corporate group involved in food and health, Meiji Group is aware of the weight of our responsibilities. With this in mind, we practice fairness and integrity based on high ethical standards and our strict adherence to Meiji Group Policy for the Marketing of Breast-milk Substitutes.
Responsibilities and Governance
The ultimate responsibility for implementing the Meiji Group Policy for the Marketing of Breast-milk Substitutes (BMS) lies with the CEO of Meiji Holdings Co., Ltd. The responsibility for the management, implementation, education, and monitoring compliance with this Policy is delegated from the CEO to the relevant executives overseeing BMS-related business units. In each country, the head of the responsible department is accountable for implementing the policy in day-to-day marketing operations and ensuring compliance in their respective regions.
Monitoring
Independently of any other measures taken by governments for the implementation of the WHO Code, we acknowledge our responsibility for monitoring our marketing practices according to the principles and aim of the WHO Code. At Meiji Group, all executives and employees involved in the marketing of covered products strictly adhere to the Meiji Group Policy for the Marketing of Breast-milk Substitutes (BMS) in their marketing conduct at every level.
Training and Communication
All executives and employees involved in the marketing of covered products receive ongoing training, which includes: the aims and principles of the WHO Code, including the importance of supporting and protecting breastfeeding, and to adhere to the Meiji Group Policy for the Marketing of Breast-milk Substitutes (BMS), as well as relevant laws and regulations in the countries where the covered products are sold.
Reporting Mechanism
In addition to normal management reporting lines in daily operations, we have established a confidential and anonymous reporting mechanism for potential non-compliance with company policy. Meiji Group has rules and regulations to protect whistleblowers from possible negative consequences of such reporting.
Compliance Counseling Desk
We maintain an internal whistleblowing system for executives and employees that includes an internal hotline as well as independent consultation desk staffed by an external attorney or other qualified professional. For overseas Group companies, we have established local reporting channels that allow executives and employees to report in the language they use. We also have a reporting channel in Japan to receive such reports.
Corrective Action for Violations
If a reported case of suspected policy violation is confirmed, it will be promptly reported to the responsible person. Once a violation is confirmed, appropriate corrective action will be taken immediately following a warning.
Product Package Labeling
The Meiji Group provides clear and accurate information in accordance with the "Meiji Group Food Nutrition Labeling Policy" on product packages to help customers choose the correct products and lead healthy lives. Product packages include mandatory labeling such as nutritional information and packaging information in compliance with the labeling regulations of each country and region where the product is sold, as well as information necessary for customers to make the correct choice.
Packaging of "SAVAS Soy Protein 100" in Accordance With the Meiji Group Food Nutrition Labeling Policy
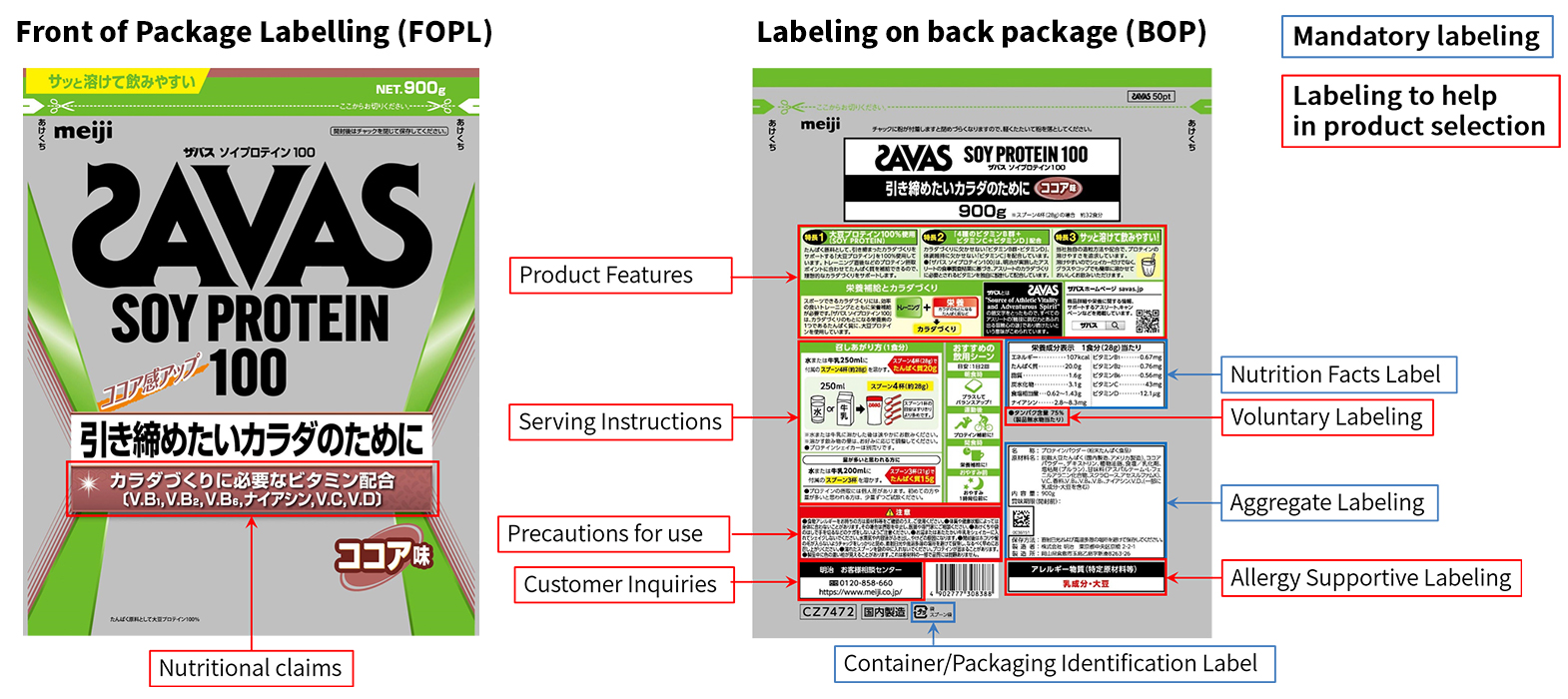
Mandatory Labeling
- Aggregate Labeling
Information on the name (product name), ingredients, content, best-before date (before opening), storage method, manufacturer, and place of manufacture are listed. In particular, many products are laser-engraved with a best-before date that cannot be altered. - Nutrition Facts Label
Calories (energy), protein, fat, carbohydrates, and salt equivalents are shown. In addition, when nutritional ingredients specified in the Food Labeling Standards are claimed to customers on the container label, the content of the nutritional ingredients is also shown. - Container/Packaging Identification Label
"Identification Mark" is displayed in accordance with the Act on the Promotion of Effective Utilization of Resources.
Labeling to Help in Product Selection
- Nutritional claims
In order to help customers correctly select Meiji Group products, the nutritional ingredients that are specifically added to our products are displayed in an easy-to-understand manner. - Voluntary Labeling
Depending on the characteristics of the product, information on ingredients considered necessary for customers is displayed near the Nutrition Facts label. - Allergy Supportive Labeling
In addition to the mandatory allergy labeling, the Meiji Group also provides a separate "Allergy Supportive Labeling" column to increase visibility. The specified raw materials and items equivalent to specified raw materials stipulated by the Consumer Affairs Agency are shown. - Product Features
Information on how the product can help customers lead healthy daily lives. - Serving Instructions
To make it easy for customers to enjoy the food, various ways of consuming the food are shown. - Precautions for use
The contents and notes of inquiries and anticipated events received from customers are shown. - Customer Inquiries
By making product package labeling highly visible and eye-catching, we strive to gain customer satisfaction and trust through the development and improvement of diet and nutrition, products and services.
Employee Education on Marketing and Advertising (Food Business)
In order to promote responsible marketing, we provide educational programs for employees involved in promotion and public relations, as well as to those who wish to do so.
We will further expand the content of our educational programs to ensure that our employees can provide the right information to customers about our products.
| Educational Program Content | FYE 3/2022 | FYE 3/2023 | FYE 3/2024 | FYE 3/2025 |
|---|---|---|---|---|
| Meiji Group Marketing Communication to Children Policy | — | 130 | 152 | 548 |
| Rules regarding the use of SDGs logos and icons in the Meiji Group | 29 | 156 | 97 | — |
Access to Nutrition
Home Delivery Service
The Meiji Group provides home delivery services for drinking milk and dairy products through a network of some 3,000 distributors nationwide, delivering directly to customers in nearly 2.4 million households. This home delivery service features numerous delivery-exclusive products that are not available in stores, including small-volume functional beverages developed to enable convenient daily consumption of products that promote health, as well as products such as cheese and curry designed to support the formation of good eating habits.
We value direct communication with our customers. We make efforts to engage with customers during deliveries and, in certain communities, also participate in neighborhood watch activities. The Meiji Group is making a shift from our previous function as delivery centers towards becoming wellness centers. We will go beyond offering products and expand our services to proposals for basic exercises as we work to extend healthy life expectancy for our communities.
 |
 |
 |
 |
| Meiji Probio Yogurt R-1 | Meiji Probio Yogurt LG21 | Meiji Dairy Five Stars | Meiji Glucosamine 1500 & Collagen 3000 |
Communicate Information on Nutrition Improvement in Emerging Countries
Many people in developing countries and emerging economies suffer from nutritional deficiencies and malnutrition.
As a company in the food business, we want to help solve these issues, working with groups that encourage nutrition improvement to raise awareness about diet and nutrition through educational activities.
Participation in the Nutrition Japan Public Private Platform
Meiji is a member of the Nutrition Japan Public Private Platform, an organization that facilitates joint initiatives in the private and public sectors to improve nutritional intake in developing countries. The platform was established following the Japanese government's declaration to step up efforts to improve nutrition worldwide. As a member, Meiji has been participating in initiatives for improving people's health and livelihoods and for ending hunger, a goal of the Sustainable Development Goals adopted by the United Nations in 2015.
Preparing for Emerging Infectious Diseases
Measures Against Emerging Infectious Diseases
Provision of KOSTAIVE®, a Self-amplifying mRNA Vaccine1 Against COVID-19
“Kostaive® for Intramuscular Injection” is a self-amplifying mRNA vaccine that can induce a non-inferior immune response even at lower doses compared to a conventional mRNA vaccine, as well as expectation for the longer duration2,3,4. In November 2023, Meiji Seika Pharma Co., Ltd. received approval for the manufacturing and marketing in Japan from the Ministry of Health, Labour and Welfare (MHLW). This is the world’s first approved product utilizing self-amplifying mRNA technology. An application for a partial amendment to target the Omicron subvariant JN.1 was approved in September 2024, and vaccinations began in October 2024.
Simultaneously, we are collaborating with ARCALIS, Inc. to establish an integrated capability for manufacturing “Kostaive® for Intramuscular Injection”. ARCALIS is constructing a drug substance manufacturing facility in Minami-soma City, Fukushima Prefecture, Japan, and Group company of Meiji Seika Pharma Co., Ltd. has been manufacturing the drug product.
Developing an Inactivated Vaccine against novel coronavirus infection (COVID-19) in Japan
We have been developing an inactivated vaccine1 (KD-414) since May 2020 in collaboration with a national research institute2, utilizing knowledge accumulated through many years of vaccine development. The clinical trials are underway since October 2021.
The multi-regional phase III clinical trials in adults under 40 years of age were started in April 2020, and the domestic phase III clinical trials in children of 6 month to less than 12 years of age were started in January 2023, respectively. In addition, in order to verify the efficacy of KD-414 against the Omicron subvariants, including XBB.1.5, we started another domestic phase III clinical trials in children of 6 months to less than 13 years of age.
In April 2025, we updated KD-414 to target JN.1 strain instead of the previous Omicron strain XBB.1.5. We are currently administering KD-414 (JN.1) to new subjects.
Creating Ground-breaking Therapeutics Using Next-generation Ivermectin Derivatives and Building a Foundation for Antiviral Drugs
Preventing the aggravation of COVID-19 infection, in addition to suppressing the infection itself is also a major issue in COVID-19 infection, and the development of safe and highly effective therapeutic agents is urgently needed. Since May 2021, Meiji Seika Pharma Co., Ltd. and the Kitasato Institute have been conducting a joint R&D project to discover ground-breaking COVID-19 therapeutics using next-generation derivatives of ivermectin and to establish a platform for antiviral agents. As ivermectin derivatives are known to have anti-inflammatory and immunomodulatory effects, they are also expected to inhibit the onset of post-infection aftereffects, which remains a concern. Additionally, Meiji Seika Pharma Co., Ltd. aims to establish a system to discover innovative therapeutic agents against various other viral infections. The project was selected by the Japan AMED (Agency for Medical Research and Development) as an R&D project in its CiCLE (Cyclic Innovation for Clinical Empowerment) program in 2020.
Developing a Novel Vaccine for Dengue
KM Biologics Co., Ltd. is developing a novel vaccine (KD-382) for dengue which is wider spread in tropical and sub-tropical climates worldwide, including the developing nations. KD-382 is a live attenuated tetravalent dengue vaccine containing each live attenuated dengue virus for four dengue serotypes 1 to 4 (DENV1-4) as the active ingredient and is expected to provide preventive effect against dengue. KD-382 has shown good immunogenicity and protective efficacy for all four serotypes with a single dose administration in the non-clinical studies.
We will develop a therapeutic agent to fight dengue fever, an infectious disease that can be a greatest global threat in the future, with support from the Strategic Center of Biomedical Advanced Vaccine Research and Development for Preparedness and Response (SCARDA) and MHLW.
Combat Antimicrobial Resistance (AMR)
A Novel β-lactamase Inhibitor for Combating Antimicrobial Resistance (AMR)
OP0595 (International Nonproprietary Name: nacubactam), a novel beta-lactamase inhibitor developed by Meiji Seika Pharma Co., Ltd., is expected to be an effective treatment for infections caused by Gram-negative bacteria resistant to carbapenem antibiotics, which are considered the last resort in the treatment of severe infections. The application for manufacturing and marketing approval in Japan was submitted in December 2025.
Providing Information on the Proper Use of Antibiotics
The Meiji Group actively engages in public awareness campaigns about medical and health issues in collaboration with related organizations. For example, the Group participates in the Japan Pharmaceutical Manufacturers Association's stewardship project that aims to help stop antimicrobial resistance (AMR). Additionally, the Group encourages medical organizations to display posters and show educational videos produced through the project, dissaminates information on drug tolerance, and promotes initiatives undertaken by the association's members. The Group also raises awareness of One Health, an approach that emphasizes the interconnectedness of human, animal, and environmental health, which is crucial for combatting AMR.

Appropriate Use of Antibiotics: Efforts to Control Infectious Diseases Caused by Vancomycin-Resistant Bacteria
The global risk of infectious diseases caused by drug-resistant bacteria* is globally increasing partly due to inappropriate use of antibiotics. One notable example is the rise in infections caused by vancomycin-resistant bacteria. The frequent use of vancomycin for treating MRSA infections has lead to confirmed increases in vancomycin resistance.
To prevent the spread of these drug-resistant bacteria, the Ministry of Health, Labour and Welfare (MHLW), the Japan Antibiotics Research Association and pharmaceutical companies established the Vancomycin Study Group. Meiji Seika Pharma Co., Ltd. has served as the organizer of the Association since its establishment in 2002, leading numerous efforts to ensure the appropriate use of vancomycin. Collaborating with relevant organizations, the Study Group continuously monitors quantities of drugs used in order to detect and understand changes or any signs of increase in drug resistance. The Study Group reports survey results to the MHLW and shares that information to medical institutions.
Antimicrobial Resistance (AMR) Survey in Industrial Animals as a “One Health” Approach
Zoonotic diseases that can be transmitted between animals and humans, account for about half of all infectious diseases. These infections are not confined to those in medical and veterinary fields, but can easily spread to the general public through the environment and food, and even internationally.
Proper use of antimicrobials in livestock farms is crucial to prevent the global spread of drug resistance in veterinary and human medical settings. Meiji Animal Health is conducting joint research on drug-resistant bacteria in industrial animals at AMR Surveillance Laboratory (AMRSL) in Azabu University. AMRSL collects specimens from infected livestock and breeding environments nationwide, investigates drug susceptibility and examines the prevalence of resistance genes in the isolated strains. By accumulating these basic data and overall profiling the susceptibility of the strains to various drugs, we will develop measures to suppress the emergence of AMR (AMR).
Stable Supply of Pharmaceuticals by Building a Robust Supply Chain
Ensuring a Stable Supply System for Antibacterial Drugs Essential for Medical Care
Production troubles at overseas bulk drug manufacturers led to disruptions in the shipment of antibacterial drugs, which are essential for medical care, to Japan. Since then, many drugs faced restrictions or disruptions of their shipments.
Additionally, when COVID-19 spread globally in 2020, officials from the Ministry of Health, Labour and Welfare created a list of essential medicines, called "Stable Supply Medicines." Of the six components in Category A, the highest priority category on this list, Meiji Seika Pharma Co., Ltd. supplies four: Vancomycin Hydrochloride, Meropenem Hydrate, Sulbactam Sodium/Ampicillin Sodium, and Tazobactam Sodium/Piperacillin Sodium.
In order to meet our social responsibility and to play a central role in the treatment of infectious diseases, we established Meiji Seika Pharmatech Co., Ltd. In 2022 to optimize our production bases. Additionally, we are proposing a "Consortium Plan" for collaboration with other companies in generic drugs to ensure quality and to strengthen the stability of our supply system.

Establishing a Domestic Production System for Penicillin Bulk Drugs, to Reduce Foreign Dependency
In December 2022, 11 materials including semiconductors and storage batteries were designated as "Specified Critical Products" by government ordinance under the Act on the Promotion of Ensuring National Security through Integrated Implementation of Economic Measures. These materials have a significant impact on the survival of citizens and on their lives and economic activities. Among these products is antibacterial drugs. The four b-lactamase antibacterial drugs that were designated are essential for the treatment of infectious diseases and the prevention of infection during surgery. The bulk drugs for these antibacterial drugs are imported from overseas.
Meiji Seika Pharma Co., Ltd. is preparing to produce 6-APA, a common raw material for penicillin antibacterial drugs, at its Gifu Plant starting in 2025, to ensure a stable supply of two of these drugs in Japan.
Domestic production of this raw material will enable us to secure a stable supply of 6- APA, which currently depends on supplies from overseas, and to establish an integrated capability for manufacturing penicillin antibacterial agents in Japan.

Establish Vaccine Production System in Preparation for Infectious Disease Pandemic Outbreak
KM Biologics Co., Ltd. has received funding through a project launched by Japan's Ministry of Health, Labour, and Welfare to ensure the development and production of vaccines for new strains of influenza. In the event of an influenza pandemic, the company is responsible for manufacturing and supplying influenza vaccine to 57 million people (almost half the population of Japan). In addition, since the project has been selected by the Ministry of Economy, Trade and Industry (METI) for the "Project of Developing Biopharmaceutical Manufacturing Sites to strengthen Vaccine Production," we will establish a vaccine production facility in preparation for an infectious disease pandemic outbreak. This development will enable the establishment of a system that can smoothly produce vaccines domestically in the event of an emergency.
Ensuring a Reliable Supply System for Sole-Supplier Products and Orphan Drugs
KM Biologics Co., Ltd. is the sole manufacturer and supplier of various sole-supplier products1 in Japan, including antivenoms for poisonous snakes (Mamushi and Habu), a hepatitis A vaccine, an anthrax vaccine for livestock, and various diagnostic agents.
he Meiji Group has received 14 orphan drug2 designations for 10 products in total, and 10 out of 11 indications (one indication/product in clinical trials) have been approved, contributing to treatments of diseases with limited treatment options (As of the end of March 2025).
Major Orphan Drugs
Refractory epilepsy: In 2012, Meiji Seika Pharma Co., Ltd. launched DIACOMIT®, a treatment for Dravet syndrome, a refractory epilepsy syndrome that develops in infancy. In 2011, DIACOMIT® received an orphan drug designation for the disease.
Brain tumors: Photodynamic therapy (PDT) is known as a less invasive treatment that has less impact on normal tissues. “Laserphyrin® 100mg for Injection”, a photodynamic therapy agent, received orphan drug designation in 2013 for the indication of primary malignant brain tumors and was approved in the same year.
Esophageal cancer: In esophageal cancer, there has been a desire for a treatment for local residual or recurrent tumors after chemoradiation therapy (CRT) or radiotherapy (RT). “Laserphyrin® 100mg for Injection”, a photodynamic therapy agent, was designated as an orphan drug in 2014 for this indication as well and received approval in 2015.
Graft-versus-host disease (GVHD): Chronic graft-versus-host disease (GVHD) is a complication with limited treatment options. It occurs after hematopoietic stem cell transplantation for hematologic cancers, such as leukemia. “RezurockÒ 200mg Tablets”, launched in 2024, is shown to be effective against this disease by exerting immunomodulatory effects through a new mechanism of action, and was designated as an orphan drug in 2023.
Infectious diseases caused by strains resistant to carbapenem antibacterial agents: “OP0595” (International Nonproprietary Name: nacubactam) is a novel b-lactamase inhibitor developed by Meiji Seika Pharma Co., Ltd. The combination with existing b-lactamase antibacterial drugs is expected to effective against antimicrobial-resistant (AMR) bacteria and is being examined in global Phase III clinical trials since May 2023. OP0595 received orphan drug designation in November 2024.
Purpura fulminans: Patients who have a congenital deficiency of a protein called protein C may develop purpura fulminans, a serious disease characterized by recurrent subcutaneous bleeding and hemorrhagic necrosis.
"Anact® C Injection 2500 units" is a preparation of purified and activated protein C from donated blood plasma, used for the treatment of purpura fulminans.
This drug has been designated as an orphan drug since 1993.
Hemophilia with inhibitors: Hemophilia patients, both hemophilia A and B, who develop antibodies (inhibitors) against Factor VIII and Factor IX involved in blood coagulation reaction may experience bleeding in joints and muscles due to impaired clotting function.
"Byclot® Combination I.V. Injection" is a preparation containing activated Factor VII and Factor X, purified from donated blood plasma, used for hemostatic treatment and bleeding prevention in hemophilia patients with inhibitors.
This drug has been designated as an orphan drug since 2014.
Guillain-Barré Syndrome (GBS): It is a neurological disorder in which the autoimmune system damages the peripheral nerves, resulting in paralysis of the hands and feet.
"Kenketsu Venilon®-I Intravenous Injection" is a preparation of purified immunoglobulin from donated blood plasma, used for the treatment of acute exacerbation of Guillain-Barré Syndrome.
This drug has been designated as an orphan drug since 1996.
Access to Medicine
Distributing the Mpox Vaccine Through the Japanese Government
In January 2025, the Japanese government supplied the Democratic Republic of the Congo with 50,000 doses of Mpox vaccine, which KM Biologics Co., Ltd. has been approved to manufacture and market. Meiji Seika Pharma Co., Ltd. and KM Biologics Co., Ltd. will collaborate with organizations such as the WHO and the Ministry of Health, Labour and Welfare to help eliminate the serious spread of the disease in African countries, particularly the Democratic Republic of the Congo, -they aim to support international public health emergency responses by expanding vaccinations in endemic areas.
Medreich Contributes to Medicine Access Through UNICEF
Medreich Limited conducts pharmaceutical manufacturing business, including CMO (contract manufacturing organization) and CDMO (contract development and manufacturing organization), in India. The company manufactures generic drugs and sells them to markets worldwide, including Europe, Asia, Africa, and Oceania. Its U.K.-based subsidiary, Medreich plc, sells pharmaceutical products manufactured by Medreich Limited to the EU and other markets. Medreich plc supplies the antibiotic amoxicillin to UNICEF. Medreich will continue improving children's access to medicine through UNICEF.
